sodium protons neutrons electrons|Protons, Neutrons, Electrons for Sodium (Na, Na+) : Clark In this video we’ll use the Periodic table and a few simple rules to find the protons, electrons, and neutrons for the element Sodium (Na). . and neutrons for the element Sodium . 20 Easy Pokémon To Draw: A List For Artists With Step-By-Step Tutorials. Drawing what you’re passionate about is the greatest way to begin started. As a result, you may keep working on your project even when you hit a creative wall. The various Pokémon created by Nintendo and Game Freak are some of the cutest animals to hone one’s drawing .
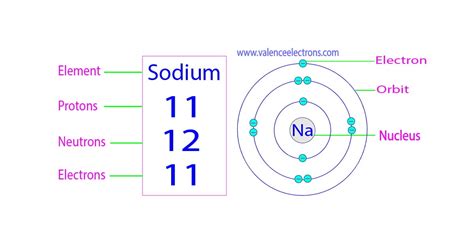
sodium protons neutrons electrons,Sodium-23 is composed of 11 protons, 12 neutrons, and 11 electrons. Acute neutron radiation exposure (e.g., from a nuclear criticality accident) converts some of the stable 23Na in human blood plasma to 24 Na.The number of protons in an atom. Electron configuration The arrangements of electrons above the last (closed shell) noble gas. . This is approximately the sum of the number of .
In this video we’ll use the Periodic table and a few simple rules to find the protons, electrons, and neutrons for the element Sodium (Na). . and neutrons for the element Sodium . Protons are found in the nucleus of the atom. This is a tiny, dense region at the center of the atom. Protons have a positive electrical charge of one (+1) ( + 1) and a .
Sodium is the 11th element in the periodic table and has a symbol of Na and atomic number of 11. It has an atomic weight of 22.98977 and a mass number of 23. Sodium has eleven .

Electrons and protons have electrical charges that are identical in magnitude but opposite in sign. Relative charges of −1 and +1 are assigned to the electron and proton, respectively. Neutrons have .Summary. Atomic Number – Protons, Electrons and Neutrons in Sodium. Sodium is a chemical element with atomic number 11 which means there are 11 protons in its nucleus. Total number of protons in the nucleus is .
Name: Sodium Symbol: Na Atomic Number: 11 Atomic Mass: 22.98977 amu Melting Point: 97.72 °C (370.87 K, 207.9 °F) Boiling Point: 883 °C (1156 K, 1621 °F) Number of Protons/Electrons: 11 Number of .
Electrons are a type of subatomic particle with a negative charge. Protons are a type of subatomic particle with a positive charge. Protons are bound together in an atom's .Number of Protons: 11: Number of Neutrons: 12: Number of Electrons: 11: Melting Point: 97.88° C: Boiling Point: 552.9° C: Density: 2.62 grams per cubic centimeter: Normal Phase: Solid: Family: Alkali Metals: Period: 3: . Sodium chloride or salt (NaCl) Soda ash (Na 2 CO 3) Baking soda (NaHCO 3) Sodium hydroxide or caustic soda (NaOH)

Protons and neutrons are found in the nucleus, the dense region at the center of an atom. Electrons are found outside the nucleus. Protons are positively charged and have a mass of about 1 u. .While protons and neutrons are located inside the nucleus at the center of the atom, electrons are located outside the nucleus in what is often called the electron cloud. Figure 4.4.1 4.4. 1: Electrons are much smaller than protons or neutrons. If an electron was the mass of a penny, a proton or a neutron would have the mass of a large bowling .Protons, Neutrons, Electrons for Sodium (Na, Na+)A proton is one of the subatomic particles that make up matter. In the universe, protons are abundant, making up about half of all visible matter.It has a positive electric charge (+1e) and a rest mass equal to 1.67262 × 10 −27 kg (938.272 MeV/c 2)— marginally lighter than that of the neutron but nearly 1836 times greater than that of the electron. Boron is a chemical element with atomic number 5 which means there are 5 protons and 5 electrons in the atomic structure.The chemical symbol for Boron is B. Significant concentrations of boron occur on the Earth in compounds known as the borate minerals. There are over 100 different borate minerals, but the most common are: borax, .For example, the atomic number of sodium is 11. Every sodium atom has 11 protons and 11 electrons. It has 11 positive charges and 11 negative charges. The mass number of an atom is its total .
The mass of a proton or a neutron is about 1836 times greater than the mass of an electron. Protons and neutrons constitute the bulk of the mass of atoms. . For example, a neutral sodium atom (Z = 11) has 11 electrons. If this atom loses one electron, it will become a cation with a 1+ charge (11 − 10 = 1+). As per the Bohr model, the sodium atom consists of 11 protons, 11 electrons, and 12 neutrons. . Number of protons. The electrons in the sodium atom are arranged in 3 shells viz. K, L, and M shell containing 2, 8, and 1 electron, respectively. The maximum number of electrons that can be accommodated in a shell is given by the formula 2n 2.For example, a neutral sodium atom (Z = 11) has 11 electrons. If this atom loses one electron, it will become a cation with a 1+ charge (11 − 10 = 1+). A neutral oxygen atom (Z = 8) has eight electrons, and if it gains two electrons it will become an anion with a 2− charge (8 − 10 = 2−). . Determine the numbers of protons, neutrons .Name: Sodium Symbol: Na Atomic Number: 11 Atomic Mass: 22.98977 amu Melting Point: 97.72 °C (370.87 K, 207.9 °F) Boiling Point: 883 °C (1156 K, 1621 °F) Number of Protons/Electrons: 11 Number of .
The atomic number of a sodium atom is 11 and its mass number is 23. Calculate the number of protons, neutrons and electrons it contains. Show answer Hide answer. Number of protons = 11. So once again for protons, we look at the atomic number, that's 92. So there must be 92 protons. In a neutral atom, the number of electrons is equal to the number of protons. So .A proton is one of the subatomic particles that make up matter. In the universe, protons are abundant, making up about half of all visible matter.It has a positive electric charge (+1e) and a rest mass equal to 1.67262 × 10 −27 kg (938.272 MeV/c 2)— marginally lighter than that of the neutron but nearly 1836 times greater than that of the electron.sodium protons neutrons electronsThe mass of an electron is very small compared to a proton or a neutron. 1 840 electrons have the same mass as 1 proton or neutron. Since the nucleus only contains protons and neutrons, most of .A proton is one of the subatomic particles that make up matter. In the universe, protons are abundant, making up about half of all visible matter.It has a positive electric charge (+1e) and a rest mass equal to 1.67262 × 10 −27 kg (938.272 MeV/c 2)— marginally lighter than that of the neutron but nearly 1836 times greater than that of the electron.
Fluorine protons neutrons electrons. Thus, the number of neutrons in an element is obtained from the difference between the number of atomic masses and the number of atoms. That is, neutron number (n) = atomic mass number (A) – atomic number (Z) Mass number (A) Atomic number (Z) Neutron number = A – Z. 19 (18.998) 9.
sodium protons neutrons electrons Protons, Neutrons, Electrons for Sodium (Na, Na+) Number of Neutrons = Mass Number - Number of Protons = 1 - 1 = 0. For zinc, the atomic weight is 65.39, so the mass number is closest to 65. Number of Neutrons = 65 - 30 = 35. Cite this Article. Follow these simple steps to find the number of protons, neutrons, and electrons for an atom of any element.
sodium protons neutrons electrons|Protons, Neutrons, Electrons for Sodium (Na, Na+)
PH0 · Sodium (Na)
PH1 · Sodium
PH2 · Protons, Neutrons, Electrons for Sodium (Na, Na+)
PH3 · How to find the Number of Protons, Electrons, Neutrons for
PH4 · Chemical Elements.com
PH5 · 4.4: Protons, Neutrons, and Electrons
PH6 · 2.6: Protons, Neutrons, and Electrons in Atoms
PH7 · 1.8: Subatomic Particles